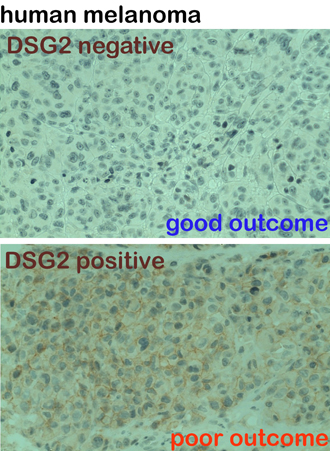
Australia has the highest rates of melanoma in the world.
Researchers at the Centre for Cancer Biology (an alliance between the University of South Australia and SA Pathology), have discovered a new protein which dramatically accelerates the spread of melanoma - a disease that is almost universally fatal if it spreads to vital organs.
Forty per cent of newly diagnosed melanomas express this protein, called desmoglein-2 or DSG-2. For people with DSG-2 on their melanoma cells, they will be two and a half times more likely to die within 10 years.
Our research team is developing new detection tools and targeted treatments that will be specifically tailored to deactivating this protein.
In fact, I’m proud to let you know that our initial lab work has shown how to identify and block DSG-2 using nanotechnology. By injecting a cancer cell seeking molecule, we can find these melanoma cells and attack them.
In Australia, every day 50 people are diagnosed with melanoma and three to four people will die. We have the target in sight but need your help to progress our important work, and we need to act fast.
We are initially seeking $150,000 to progress our pre-clinical laboratory work. When you donate today, 100% of your support will enable us to develop innovative treatments to deactivate this protein and stop the cancer.
Your donation will make an impact:
![]() $70 provides an hour of research
$70 provides an hour of research
![]() $700 a full day of research
$700 a full day of research
![]() $1,000 goes toward developing a histology test to inform a patient if their melanoma is DSG-2 positive or not.
$1,000 goes toward developing a histology test to inform a patient if their melanoma is DSG-2 positive or not.

UniSA’s Bradley Building
UniSA’s cancer research brings together more than 300 leading biomedical, genetic and pharmacological experts located in the UniSA Bradley Building on North Terrace. With so many minds at work, the effort is moving fast, and yet, for many cancer patients, it’s still not fast enough.
Although extraordinary advances have been made through groundbreaking research, cancer continues to exert an enormous toll. We need your help to be in a position where we can bring our world-leading discoveries and treatments to patients sooner so we can save lives faster.
Immunotherapy trials target aggressive tumours
Dr Tessa Gargett is an early career researcher whose work in immunotherapy is achiving significant impact for patients fighting aggressive cancers.
Soon her findings will be applied to treating children and adults with deadly brain cancers.
Dr Gargett is part of a team of researchers led by Professor Michael Brown, working within the Centre for Cancer Biology and working in collaboration with the Cancer Clinical Trials Unit at the Royal Adelaide Hospital.
Professor Brown's team is one of only two teams in Australia conducting CAR T cell immunotherapy clinical trials in patients with difficult-to-treat solid cancers.
The team is currently running a clinical trial in melanoma at the Royal Adelaide Hospital, which will extend to sarcoma and breast cancer later in the year.
Tessa is focusing her work on the highly aggressive glioblastoma and DIPG brain tumours, which are heartbreaking for families as currently very little can be done to save the young children and adults who are diagnosed with these cancers.
Once the safety of using this treatment on brain tumours is confirmed, the next step will be to trial the therapy on patients at the Royal Adelaide Hospital and the Sydney Children's Hospital Kids Cancer Centre in 2020 and to give hope to families faced with this deadly disease.

Lifesaving new therapies for children with leukaemia
The research team led by Professor Shudong Wang at UniSA is a world-leader in developing effective and minimally toxic, targeted cancer therapies. They are currently fine-tuning a safer therapy for children with acute leukaemias to improve survival rates and reduce side effects.
“Our approach to creating better cancer therapies is to find and then target the specific genes or proteins that drive cancer cell development and growth,” says Professor Wang, Head of the Drug Discovery and Development research group.
“Our new targeted therapies will reduce the side-effects caused by chemotherapies to safeguard the developing minds and bodies of children with leukaemia.”
Professor Shudong Wang, an internationally recognised leader in cancer drug discovery and development
“By targeting these specific genes or proteins, our drug molecules are more effective against cancer cells but less toxic to normal tissue. So our therapies are very different to the conventional chemotherapies that kill both cancer and healthy cells.”
Acute leukaemia is the most common form of childhood cancer representing a third of malignancies. It is the primary cause of childhood cancer-related mortality.
The more dangerous forms of acute leukaemias share a mutated gene called the mixed lineage leukaemia (MLL) gene. Survival rates are bleak for these patients and highly intensive chemotherapy is often used to try to stop the cancer. Unfortunately, these treatments often leave children with severe side effects that can persist into adulthood.
“We are investigating a specific class of cancer causing proteins called cyclin-dependent kinases (CDKs)that drive cancer cell survival and growth.
“We have developed a highly potent and selective CDK inhibitor drug that can effectively block the cancer from continuing to grow and kill the cancer cell with minimal toxicity to healthy cells.
“This provides a very exciting prospect for treatment of childhood leukaemia; especially as the drug compound can be given orally and improve the protection of the vitally important development stages of their young bodies, which current treatments often harm.”
Professor Wang’s research team is also developing several other new therapeutics that effectively target other types of blood cancers and solid tumours of the breast, prostate and ovary.

New hope for brain cancer patients
World-leading brain cancer researchers based at UniSA have developed new targeted drug therapies that could drastically improve treatment for lethal brain cancers, particularly glioblastoma.
“Our research has made an important advance for improving treatment for aggressive brain cancers.”
Professor Stuart Pitson, an internationally recognised leader in brain cancer research
“Glioblastoma is notoriously hard to treat with radiotherapy and chemotherapy. But the cancer also sends finger-like tendrils into vital brain structures, so surgery can usually only remove less than 90% of the tumour,” says Professor Stuart Pitson, Head of the Molecular Signalling Lab in the Centre for Cancer Biology.
“These cancers often regrow within six to nine months – obviously they have devastating outcomes and effective new treatments are desperately needed.
“We have identified what we call a ‘survival protein’ that protects glioblastomas from treatment and helps them grow. We are studying it to understand how we can overcome it to make chemotherapy and radiotherapy treatments more effective.”
This work has also allowed the team to identify a therapy candidate molecule that can bind to this survival protein and stop it in order to make the brain cancer cells more vulnerable to chemotherapy.
“Our pre-clinical studies have been extremely promising and are also showing potential as a new treatment for medulloblastoma – the most common brain tumour affecting children.”

Innovation to predict best treatment for cancers
Using advanced computing simulation, UniSA’s worldleading radiation oncology and radiobiology researchers can analyse and predict the best combination of treatment for hard to treat cancers.
Professor Eva Bezak leads this work at UniSA, “Our in silico (in a computer) models allow us to grow biologically correct tumours that represent a patient’s cancer, to measure how quickly it will grow or how aggressively it may spread.
“Importantly our model can personalise the dose of treatment to the individual’s unique radiobiological profile. For example, we could take a simple blood and hair sample from a patient, expose them to different radiation doses and investigate the patient’s individual response to radiation damage – their radiosensitivity
“This sensitivity data can then be put back into the model so we can then run a series of simulations with not only radiotherapy doses, but also chemotherapy and other therapies to determine the very best combination and doses of treatment to improve the patient’s chance of successful treatment.
“If we can predict the best treatment combination, schedule and dose that can target difficult to treat cancers while sparing healthy cells – that to me is a successful step towards winning the war on cancer.”
Professor Eva Bezak, one of Australia’s leading experts in clinical radiation biology research
“With research providing a deeper understanding of cancer biology, the scientific community is starting to realise that all cancers are “rare” or genetically diverse. There are many different genetic subtypes that respond differently to treatments and the one size fits all treatment model is becoming outdated.
“Our model also gives us the opportunity to investigate lower doses that will deliver the maximum blow to cancer cells while limiting the amount of damage to healthy cells. This is particularly important for cancers like HPV positive head and neck cancer.
“Computational simulations could also offer hope for people with rare and difficult to treat cancers like those of the brain, as well as many others, as we can design more appropriate treatment regimes.”
Professor Bezak and her team are leading the world in this area of radiobiology cancer modelling and are aiming to create a practice where this modelling can be commercially available and in use within several years.


Fast-tracking prostate cancer breakthrough
A team of world-leading prostate cancer investigators at the UniSA Cancer Research Institute, led by Professor Doug Brooks, has tracked not only how the cancer cells talk to each other, but also how they send out beacons filled with their basic needs to aid in its spread.
Professor Brooks' team has used this knowledge to create new diagnostic tools for prostate cancer and other common cancers, as well as using this communication pathway to create targeted treatments.
With more than 98 per cent accuracy, his team has created a prostate cancer test that can tell us if the cancer will be aggressive or not so that the very best treatment option can be prescribed
And soon this research will be tested for its real world impact for prostate cancer sufferers, with a clinical trial scheduled to commence later this year.
Donations made to the Fight Against Cancer fund last year helped purchase the very latest tissue staining technology (a Roche-Ventana Ultra Tissue Stainer) which is essential for high volume diagnostic analysis and is enabling the team to get to trials faster and help patients sooner.
“Currently, cancer doctors must rely on flawed PSA tests to diagnose prostate cancer and predict how the cancer will grow,” says Professor Doug Brooks, Head of the Mechanisms in Cell Biology and Diseases Lab at UniSA.
"Up to 50% of men have false-positive PSA test results. Consequently many men are sent to hospital for the second stage of tests – invasive prostate biopsies – when they do not actually have the cancer.
“The current tests also fail to identify as much as 15% of aggressive prostate cancers. These men are usually not diagnosed until after their cancer has grown into a far more deadly stage of the disease.
“PSA tests also fail to distinguish between aggressive and nonaggressive tumours, which does not help when trying to make decisions on if and how to treat a patient.
“With our new biomarkers we have developed a series of effective new tests that will transform how prostate cancer is detected and how the patient’s chance of survival and treatment plans are measured."
Starving aggressive breast cancer
Starving the blood supply to breast cancers through inhibiting a growth factor may prove to be the answer to preventing breast cancers growing into highly aggressive and fatal forms of the disease.
Cancers need a blood supply to grow and spread. Without blood they cannot get the oxygen or nutrients they need to survive.
“Breast cancers convince nearby blood vessels to bring them a blood supply, but the highly aggressive breast cancers are also very skilled at building their own blood supply structures,” says Associate Professor Claudine Bonder, Head of the Vascular Biology Lab in the Centre for Cancer Biology at UniSA and SA Pathology.
“These cancer formed blood supply structures are particularly difficult to destroy with current therapies. Roughly 30% of the blood supply to these fatal breast cancers is made up of these structures.”
A specific growth factor (a substance that stimulates cell growth and survival) is involved in both hijacking existing blood vessels and building these cancer formed blood structures to support cancer growth.
“We are testing a product that can bind to this growth factor and silence it which starves the cancer of all blood.”
“Our research may soon lead to a life-saving new treatment for aggressive breast cancers.”
Associate Professor Claudine Bonder, a leading vascular biology specialist.
This work may lead to an effective new therapy to save the lives of women and men with the most aggressive breast cancers and may provide answers for other difficult to treat cancers like aggressive melanoma.
Using gold nanoparticles to cripple cancer
Patent-pending gold nanoparticle technology under development is offering an exciting new approach to stop cancer in its tracks and revolutionise the way cancers are treated.
Led by Associate Professor Ivan Kempson from the Future Industries Institute at UniSA, the research has discovered a way to cripple a major DNA damage repair mechanism that cancers use to recover between doses of radiotherapy and chemotherapy.
“All cells have this DNA damage repair ability, including cancers - which is a major treatment issue in the fight to cure cancer, particularly metastatic cancers that have spread to other parts of the body and are much more aggressive,” says Associate Professor Ivan Kempson.
“Radiotherapy and chemotherapy work by delivering a toxic blow to cancer cells to damage them repeatedly until they die. Unfortunately healthy cells are also damaged and they require time between treatments to recover.
“Our gold nanoparticles offer a radical new approach to stopping cancer in its tracks.”
Associate Professor Ivan Kempson, Future Industries Institute.
“We want healthy cells to repair between treatment sessions but we need to stop the cancers from having the same opportunity to repair.
“Using our discovery to target this biological pathway, we have an opportunity to deliver a potent co-therapy that can stop cancer cells accessing their repair mechanism.
“The therapy would be delivered as tiny gold nanoparticles specifically designed to ensure they are targeted for delivery directly into cancer cells and not circulated throughout the body.
“We have previously shown that our gold nanoparticles also enhance the ability of radiotherapy to kill cancer cells so our approach could provide a double hit that would reduce the amount of treatment required, thus reducing side effects and costs and increasing the chance of successful treatment.
“It also offers new hope for improving the survival rates of metastatic cancers.”

Safeguarding fragile bonesduring chemotherapy
The Bone Growth and Repair Research Lab is striving to prevent bone damage and bone growth defects for child cancer patients, a common side effect of intensive chemotherapy.
This research has revealed important processes within cells that contribute to bone damage during chemotherapy. Now the team is seeking the best natural supplements to prevent bone and bone marrow injury during treatment.
“Despite cancer treatments improving, and survival rates increasing, there are still no therapies to protect children’s bones during cancer treatment,” says Professor Cory Xian, Head of the Bone Growth and Repair Research Lab.
“Children’s bones are quite different to adults because they are still going through critical stages of development. Their bones can be significantly weakened or their growth impaired from the damage caused by cancer treatments.
“Our research will reduce the painful and life limiting side effects children can experience after treatment for cancer.”
Professor Cory Xian, a global leader in bone research.
“Alarmingly we are seeing an increase in childhood cancer survivors with painful bone problems including multiple fractures, bone pain and early osteoporosis, that are linked to long-term damage from chemotherapy. Preventive therapies are urgently needed.”
Finding the right therapy is a complex puzzle. With the growing interest and affordability of alternative treatments, the research team is exploring supplements that could be used alongside chemotherapy, including fish oil, genistein and resveratrol. They are now working to understand the right dose and whether their use causes any adverse reactions when taken with chemotherapy.
“We have also found that an extract from a species of the yam plant increases the anti-cancer effect of certain chemotherapies while also improving the body’s ability to protect healthy tissue from the damaging effects of chemo.
“Our research will help provide the information we need to translate protective natural therapies into a clinical setting so that children with cancer can go on to live pain and symptom free lives.”

Understanding cell survival and death to fight against cancer
Investigations to find the key genes and proteins that cancer cells use to stay alive is revealing new information critical for the development of new cancer treatments.
“Identifying the finely tuned mechanisms that allow our bodies to function normally is also the key to finding the molecules responsible for cancer and other diseases,” says Professor Sharad Kumar AM, Co- Director of the Centre for Cancer Biology and a world-leading cancer scientist.
Professor Kumar’s discoveries of how cells die and what makes them live in cancer have resulted in multiple breakthroughs toward a better understanding and new treatments for this devastating disease.
“Our bodies consist of more than 35 trillion cells and everyday 50-70 billion die because they are old, infected or damaged. We study how cells die and why cancer cells acquire the abnormal ability to live. We have defined many parts of the cellular machinery required for this process.
“We have also found that one such protein involved in cell death, named Caspase-2 and initially discovered in our laboratory, acts as a suppressor of cancer by selectively killing damaged cells. If not removed efficiently, such damaged cells can acquire additional DNA defects that can give rise to cancer.
“As fundamental scientists we make discoveries that can add vital information to the pool of knowledge required to beat cancer. New discoveries about cancer result in a better understanding of the complexity of this disease and inform ways to design patient-specific treatments.
“Our work is unveiling vital new knowledge for the fight against cancer.”
Professor Sharad Kumar AM, Co-Director of the Centre for Cancer Biology.
“Since resistance to normal cell death is an important hallmark of cancer initiation and progression, designing ways to overcome such resistance is being used to design new anti-cancer drugs.”


Launching an immune attack on melanoma
UniSA researchers have developed a powerful new vaccine technology that could soon be used to stimulate the immune system to strike a killer blow against melanoma.
The research team are worldleaders in creating effective vaccine delivery systems – the tools required to successfully deliver vaccines into cells to provoke an immune response to disease.
“Our work in mosquito-borne disease vaccines is proving suitable as an immunotherapy for melanoma,” says Professor John Hayball, Head of the Experimental Therapeutics Lab.
“Immunotherapies that teach the immune system to attack cells based on the presence of certain molecules are highly sought after for cancers. Our treatment vaccine will be loaded with a substance to stimulate the immune system to target and destroy melanoma cells.
“Our new vaccine technology will teach the immune system to launch an effective attack on melanoma cells.”
Professor John Hayball, a leading immunologist working in disease control.
“Skin can also elicit its own anti-tumour response by activating special immune defending T-cells. To capitalise on this extra immune response, our vaccine will be delivered by a small micro-patch, similar in appearance to a small round band-aid, but with a rough surface that injects the vaccine into the skin
“These types of therapies can eliminate tumour cells while sparing healthy cells thus minimising the side effects that often plague cancer patients and survivors.”
This ground-breaking vaccine technology could also offer immunotherapy for other diseases, such as prostate cancer.
Smart device to detect skin cancer
An innovative research team at UniSA has invented a game-changing device that any doctor will be able to use to quickly check a patient’s skin for suspected skin cancers.
Created by Professor Tarl Prow and his team, the micro-biopsy device takes a tiny pinprick sample from the skin that captures roughly 200 cells. It avoids the need for painful biopsies that require short anaesthetic surgery, stitches and often leave scars of 2-3cm in length.
“Our device will make it easier to identify which skin cancers require immediate removal for further testing and which ones can be monitored over time if they pose no major threat,” says Dr Miko Yamada, Research Fellow in the Future Industries Institute.
In Australia more than 1,000,000 patients see their doctor for skin cancer consultations each year. Around 750,000 of these patients have a non-melanoma cancer treated.
“We are now fine-tuning our skin cancer biomarkers so the device can take samples and deliver the results instantly. It has the potential to save considerable time, pain and money.”
The device is now in final clinical trials and will soon be available worldwide.
“I want every GP to have one of these new skin cancer devices. They can click it on the skin and after a quick analysis, tell the patient if they have a skin cancer or not.”
Dr Miko Yamada, Research Fellow from the Future Industries Institute, pictured with Professor Tarl Prow.

New hope for identifying early-stage ovarian cancer
An early detection test for ovarian cancer is under development at UniSA. The test offers a chance to improve the survival rates for the more than 1,500 women diagnosed each year in Australia.
“Ovarian cancer is a rare but often deadly disease as it has thus far proven extremely difficult to identify and treat early,” says Professor Peter Hoffmann, an internationally recognised expert in the study of proteins and the discovery of biomarkers for early disease detection in cancer.
“We have identified a number of autoantibodies produced by the immune system at the early stage of ovarian cancer that offer high accuracy as a biomarker test for detecting early-stage ovarian cancer.
“This research resulted from almost a decade of collaborative research with Professor Martin Oehler at the Royal Adelaide Hospital that included validating our autoantibody test on 320 ovarian cancer patient samples and the results are very promising.
“We are in the process of validating a promising new early detection test for ovarian cancer to improve the chance of survival for women with this disease by finding it early.”
Professor Peter Hoffmann, Strand Leader for Biomaterials Engineering and Nanomedicine in the Future Industries Institute.
“If ovarian cancer is detected early it can often be cured. Unfortunately for many women diagnosed with the disease, it is only detected once it has spread through the abdominal cavity and has become very difficult to treat.
“The next step of our research includes larger trials with over 1,000 patients. Oncethis is complete, we expect to be able to roll it out across multiple hospitals for even larger scale trials.
“This research could very well lead to a population wide screening test to identify women at risk of developing ovarian cancers.”


Improving the lives of childhood brain cancer survivors
A specific protein may hold the key to reversing the development of medulloblastoma, the most common brain cancer in children.
Although survival rates for medulloblastoma have improved slightly in recent years, current treatments need an intensive combination of chemotherapy, radiation and surgery, which can damage critical stages of childhood development.
“We have had a breakthrough in understanding how medulloblastoma forms in the growing brain,” says Associate Professor Quenten Schwarz, Head of the Neurovascular Research Lab in the Centre for Cancer Biology at UniSA and SA Pathology.
“Current treatments can lead to lower life quality for children who survive this disease as they require highly toxic drugs that also affect the growth of normal brain tissue, so better treatments are urgently needed.
“Our team has found that if we can reverse the effects of a specific protein that becomes out of control in normal brain cell development, we can control the mutated process that leads to medulloblastoma.
“Our discovery could be the missing key to finding a better treatment for medulloblastoma – the most common form of brain cancer in children.”
Associate Professor Quenten Schwarz, an award winning investigator of human and disease development.
“By understanding more about the disease process, our work could also lead to the development of a therapy that targets and blocks this protein to actively kill medulloblastoma cells and reduce damage to healthy tissue in the brain.”
A pain free test for bladder cancer
A novel cancer diagnosis tool is under development at UniSA, which could lead to a pain free test for the detection of urothelial cancers of the bladder and urinary tract.
“We have created a highly effective new medical surface coating, that is showing extremely high ability to capture cancer cells that are shed in urine,” says Dr Melanie MacGregor, a lead researcher working alongside Professor Krasimir Vasilev in the Future Industries Institute.
“The device we have created uses a new plasma polymer compound to create a simple urine test for urothelial cancers to reduce the need for current invasive and often painful testing methods.”
“Our new bladder cancer diagnostic device holds the potential to create simple urine tests to detect many different types of cancer.”
Dr Melanie MacGregor, Research Fellow, Future Industries Institute.
Cancers of the urothelial tract have high mortality rates due to a lack of effective diagnostic tests to catch the cancer early. These cancers also have a high chance of returning or spreading to other parts of the renal tract, so cancer survivors require frequent, ongoing surveillance.
“Currently many of these patients and survivors must have regular tests every year. The tests are expensive and often invasive, such as cystoscopy – where a thin camera tube is inserted into the urethra.
“New clinical tools are urgently needed to improve both early diagnosis of these cancers and reduce the need for such uncomfortable tests.
“Our new device is an important advance in medical diagnostics. It is proving to be highly effective at capturing cancer cells in urine and has attracted considerable industry support.
“With further development it could be applied to other cancers that shed cells in urine including prostate cancer.”


Delivering chemotherapy directly into breast cancer cells
Using cutting edge nanotechnology, a team of researchers at UniSA are working to target a potent cancer therapy directly into breast cancer cells. Improving the delivery of the drug will drastically reduce the damage inflicted on healthy cells and enhance its ability to destroy tumours.
“Breast cancer is one of the most diagnosed cancers in women and while many patients now survive the disease, treatment side effects can cause ongoing health issues,” says Professor Sanjay Garg, Director of the Pharmaceutical Innovation and Development research group.
“One of the biggest challenges for the scientific community in regards to anti-cancer drugs, is fine-tuning the delivery of the therapies we know work well to the cancer site to reduce damage to healthy tissue.
“Nanomaterials offer an exciting new opportunity to target medicine; they can be loaded with potent therapies and key molecules that direct their release only when they connect with the lock molecule on cancer cells; they are also extremely small and can pass through holes in the tumour membrane that other molecules cannot enter.
“We are using cutting edge nanotechnology to enhance a potent chemotherapy and target its delivery directly into breast cancer cells to reduce toxic side effects.”
Professor Sanjay Garg, an internationally recognised pharmaceutical scientist.
“In one of our projects, run by PhD scholar Candace Day, we are loading an effective chemotherapy for breast cancers into a promising new nanomaterial called mesoporous silica nanoparticle.
“Our approach holds considerable promise as an improved, targeted therapy for the most common breast cancers with drastically reduced toxicity to healthy cells and fewer side effects.”

A simple approach to wipe out skin cancer
A team of researchers at UniSA are developing a revolutionary new approach for the treatment of skin cancers.
Led by Professor Allison Cowin, the team have discovered that a protein that affects the way our body heals is hijacked by skin cancer tumours to grow and spread.
They have also identified antibodies that can bind to the protein and neutralise it to prevent and treat skin cancers.
“We were looking at how to improve the healing of wounds by manipulating the Flightless I protein when we discovered that it also plays a role in skin cancers,” says Professor Cowin.
Australia has the highest incidence of skin cancer in the world. The cost of diagnosis and treatment is enormous – around $500 million every year. Effective new therapies are urgently needed.
“We are close to finalising a potent new therapy that will provide a simple treatment for skin cancers.”
Professor Allison Cowin, Future Industries Institute.
“Together with cancer experts and pharmaceutical scientists we are developing a monoclonal antibody therapy for the treatment of skin cancers.”
Monoclonal antibody therapies deliver specific therapeutic molecules that can bind to a protein and block their ability to work in a certain manner. In this case, blocking the Flightless I protein from helping skin cancer cells to grow and spread.
“We are looking forward to assessing this new skin cancer treatment in patient clinical trials.”
Pinpointing cancer spread during surgery
A new device developed at UniSA is helping cancer doctors pinpoint the accuracy of surgery to remove cancers that have spread into other areas of the body.
This revolutionary new device called the Ferronova Probe will solve a clinical problem in the successful treatment of cancers using magnetic tracers.
“Current procedures to find cancers that have spread through the lymphatic system into lymph nodes include injecting radioactive tracers into the tumour area that can be used to find the migration paths of cancer cells,” says Dr Aidan Cousins, who leads the project alongside Professor Benjamin Thierry from the Future Industries Institute.
“There are problems with this approach due to the limitations of current technologies and the complexity of how lymph nodes are used by different cancers to spread.
“We have developed a revolutionary new clinical tool that will improve the accuracy of surgery for the removal of metastatic solid cancers.”
Dr Aidan Cousins, Research Associate in the Future Industries Institute.
For example, some cancers, like in the oesophagus or oral cavity, may not spread very far from the tumour, and can be lost amongst the background ‘noise’ of radioactivity in the injection site.
“Our magnetic tracers allow surgeons to locate where cancers have spread within millimetre accuracy, both improving the result of surgery and reducing the need for further operations.
“The magnetic tracers we use are also cheaper and have longer shelf-life than radioactive ones. This means that more smaller and regional hospitals could use the technology to save patients travelling to larger cities for treatment.”
This device will begin clinical trials for head and neck cancers in late 2018. It holds the potential to transform clinical procedure by creating a much more targeted approach to tracking cancer spread.

Blocking aggressive breast cancers from spreading
A key ingredient that breast cancers use to grow and spread into other parts of the body could lead to the identification of a targeted drug for difficult to treat cancers such as triple negative breast cancer.
It could also provide a way to identify which women are more likely to develop these often fatal diseases.
“Our investigation of a protein called Pez (or PTPN14) has confirmed that it is expressed less or in a mutated form in highly aggressive breast cancers,” says Associate Professor Yeesim Khew-Goodall, Head of the Cell Signalling Lab in the Centre for Cancer Biology at SA Pathology and UniSA.
“We know that this protein plays an important role in cancer metastasis (the spread of cancer to other organs). Through our work we also know that mutations in this protein are just one part of a complex interaction with other proteins that promotes this cancer spread.
“This work has allowed us to successfully map an entire signalling pathway that appears to also play a key role in these fast moving, aggressive breast cancers.
“Our goal is to understand exactly how these molecules are interacting to increase cancer progression and identify new therapies that can create better outcomes for these patients.
“Our work could help save the lives of cancer patients with highly aggressive, fast growing breast cancers.”
Associate Professor Yeesim Khew- Goodall, a leading researcher in the drivers behind cancer metastasis.
“One of the most promising aspects of our discovery is that the pathway has components that are potentially “druggable”, meaning there is an essential component of the pathway that we expect to be able to devise a very specific drug for. This will be our next step.”

Fighting bowel cancer with Big Data
A team of researchers at UniSA are hunting through millions of data records to find the hidden trends that could lead to improved health system performance and markedly cut bowel cancer deaths.
Australia has one of the highest rates of bowel cancer in the world. Professor David Roder and his team are working to improve the survival rate of this disease.
“We believe we can drastically reduce the number of deaths from bowel cancer in Australia by 2030.”
Professor David Roder, a world-leading cancer epidemiologist guiding national and international best practice in cancer treatment and prevention.
“We believe we can help our health system to significantly reduce deaths from bowel cancer in Australia by 2030,” says Professor David Roder, Chair of Cancer Epidemiology and Population Health at UniSA.
“We are working as part of a collaborative team of researchers, policy makers and care providers to find the scientific evidence we need to ensure only the very best treatments and services are provided to bowel cancer patients.
“In South Australia, our bowel cancer care system and survival rates are very high by international standards, so we have an opportunity to compare and learn what we are doing here that could be applied elsewhere.
Data suggests that there are certain factors that increase the risk of death from a bowel cancer diagnosis, including living in remote areas, having other complex health conditions and low socio-economic status.
“By exploring patient data from cancer registries, hospital records, treatment centres, health insurance and screening registries, we can provide opportunities to strengthen services and address gaps to ensure impact where it is most needed to create a much more targeted approach to treating bowel cancer.
“There is a science to understanding and using science to improve healthcare. One of the difficulties in ensuring patients get the very best treatments is making sense of the immense amount of research data. This is what we strive to achieve in our work.”

Preventing aggressive breast cancers with vitamin D
Vitamin D, that sunny little hormone that supports healthy bones and overall wellbeing, could play an important role in how breast cancers form and metastasize (spread into other parts of the body).
“We are looking at the strong link between vitamin D deficiency in breast cancer cells and their growth into neighbouring organs like the lungs,” says Associate Professor Paul Anderson, Head of the Musculoskeletal Biology Research Lab at UniSA.
“Our research into vitamin D deficiency in breast cancer cells could lead to strategies to better understand and prevent breast cancer"
Associate Professor Paul Anderson, Head of the Musculoskeletal Biology Research Laboratory
“This analysis could give us additional clues as to why some breast cancers form in the first place and offer strategies to prevent breast cancers.”
The kidney is normally considered the major organ for producing vitamin D, but Associate Professor Anderson and his team have identified that a variety of cells also produce it for their own purposes, including mammary cells in the breast.
“This production of vitamin D appears to support cell differentiation and other normal cellular processes that stop cancer forming,” he says.
“If this process within cells is interrupted or blocked for some reason, as we have seen in our analysis of breast cancer tissues, then there may be a higher risk of breast cancer developing and then spreading more aggressively to other parts of the body.
The research team have undertaken RNA analysis on both healthy and cancerous breast tissue samples to identify which genes are disturbed by altered levels of vitamin D.
“We found a large number of genes were changed in vitamin D deficient breast cancer tissue samples, including four genes that are known to act as tumour-suppressors.
“Our next step will be to explore what levels of vitamin D need to be maintained within breast cells as an effective cancer treatment and prevention therapy.”
The Musculoskeletal Biology Research Lab is working on this research in collaboration with the University of Adelaide and McGill University in Canada.

Unveiling the challenges of life after cancer
Cancer care researchers at UniSA are leading Australia toward including a standard quality of life assessment in routine monitoring of cancer survivors to help them go on to live the very best lives they can after a diagnosis.
During cancer treatment there is an intense focus on survival; on beating the cancer so that life can continue. Patients are busy attending chemotherapy and radiotherapy appointments, visiting specialists, and coping with the physical, emotional and practical strain of their diagnosis. Throughout this time their care is carefully managed by a dedicated health team.
When treatment ends, if no ongoing therapy is required, patients can suddenly feel cast adrift as they are expected to return to their old lives. Yet life has changed.
Until recently, the quality of how a patient was supported after cancer stretched as far as any major complications or risk of the cancer returning. But as cancer treatments have improved, cancer survival rates have followed and today there are an estimated 1.1 million cancer survivors in Australia – many living with a range of physical, emotional and practical issues.
“We are addressing the gaps in care that cancer survivors can experience after they finish treatment"
Professor Marion Eckert, Inaugural Professor of Cancer Nursing in South Australia
“What we need now in Australia is a gold-standard measurement in our public health system of how these people are living, what extra care or services they might need to really live a happy and healthy life after cancer treatment, and to identify those people most at risk,” says Professor Marion Eckert, Director of the Cancer Care Research Group.
“We know that there are a range of issues that can arise – physical side-effects from treatments, financial difficulties, strain on mental health and relationships for example – there are a number of areas in these people’s lives that will change and they may require help to manage.
“The problem is that either no system is set up to capture this information, or where they are in place they are usually different, so these systems don’t always link and therefore the impact is difficult to measure.
“This means that we do not have a deep, long-term understanding of the health and wellbeing trends of cancer survivors; so it is difficult to gather the evidence required to create new standards of care that every patient can access.
“Our team is working with Australian and international leaders in this area. Our colleagues in the Netherlands for example are world-leaders in understanding cancer survivor needs and implementing services to support them.
“We are currently developing CanLead – a research program to inform a gold-standard measurement that will capture the burden of health and wellbeing gaps in care for cancer survivors.
“Much work has been done internationally to systematise, standardise and in nearly all countries, legislate the routine reporting of a cancer diagnosis. So we can quite easily tell you how many people are living with cancer. What we can’t tell you, is how well they’re living – which this research will help change,” says Professor Ian Olver, Director of the UniSA Cancer Research Institute.

A new frontier of statistical research – whole genome risk screening
Dr Sang Hong Lee, a statistical geneticist leading a team in the Australian Centre for Precision Health, is forging a new path in biostatistics and AI driven mathematical computer applications that may soon be able to scan the entire genome to find disease risks like cancer.
“The genomic era we are now in gives us a real opportunity to find people at high-risk and low-risk of poor health outcomes, which will help us better understand what causes disease,” says Dr Hong Lee, Senior Lecturer in Statistical Genetics at UniSA.
“In the future it may be possible to predict future disease risk at birth, with the potential to predict and intervene in order to prevent the progression of disease.
“However, the accuracy of this level of risk prediction using current technologies is not yet good enough to allow it to be applied as an intervention for complex diseases like cancer.
“We are at the forefront of using advanced computing and statistics that can scan the whole genome to find the best opportunities to prevent cancer and other diseases.”
Dr Sang Hong Lee, a leader in the field of statistical genetics and disease risk analysis.
“My team are using advanced statistics to create intelligent computer models capable of scanning the whole genome to predict individual risks with far greater accuracy.
“It will also allow us to create a better understanding of the shared genetic and environmental architecture between different types of diseases and conditions – this novel knowledge can be used in a wide range of clinical practice, e.g. individual risk for a disease (cancer) can be intervened by modifying other traits that are associated with the disease.”

Preventing cancer before it strikes
The future of precision medicine—also known as personalised medicine—is speeding toward reality with the vast quantities of health data that is now available for scientists to explore. Researchers at the Australian Centre for Precision Health at UniSA are now extending this approach to disease prediction and prevention, aiming to prevent cancer before it strikes.
“With advances in our scientific understanding of DNA, and the ‘big data’ available to us now about people’s genes, lifestyles and health factors, a new world of population level analysis is available to us,” says Professor Elina Hyppönen, Director of the Australian Centre for Precision Health.
Research led by Professor Hyppönen and her team is using data from cohorts of up to 500,000 volunteers to pin-point the genetic and environment risk factors (also called exposures) that can lead to cancer or other complex diseases and conditions.
“This precision health approach is about identifying the ways in which we have an opportunity to prevent disease from happening, rather than focusing on curing it,” she says.
“With our research, in future people at risk of disease could be found earlier and prevention methods applied before they develop cancer or other health complications.”
Professor Elina Hyppönen, a world-leader in population based disease risk discovery.
“We work to construct individual risk factor profiles based on genetic data, and extensive information on environmental and lifestyle exposures. This can help us make tailored prevention strategies, which are likely to work for a particular individual.
“Ultimately, our goal is to prevent disease before people even realise they are at risk.
“One part of our work aims to establish all the possible health effects or diseases that are caused by selected exposures, which could be drugs or modifiable lifestyles.”
Professor Hyppönen’s work is exploring a number of areas that could shine light on cancer risk such as blocked vitamin D metabolism, high coffee consumption, or physical activity.
“Traditional research is hypothesis based and is limited by human knowledge. With our methods we are able to move toward a data driven approach that shows us where we need to concentrate our research and prevention efforts to reduce the impact of disease on society.”

Get in touch with our Advancement Services team by email, or contact a specific staff member
Contact the Advancement team on +61 8 8302 7375 or email us

UniSA respectfully acknowledges the Kaurna, Boandik and Barngarla First Nations Peoples and their Elders past and present, who are the First Nations’ Traditional Owners of the lands that are now home to our campuses in Adelaide, Mount Gambier and Whyalla.
ARTWORK: Ngupulya Pumani
The University of South Australia is a Deductible Gift Recipient (DGR).
Copyright Privacy notification Web accessibility Disclaimers
CRICOS provider number 00121B Australian University provider number PRV12107